Aviation Carbon Monoxide poisoning is a safety issue that pilots tend to ignore and it is an overlooked safety issue. When carbon monoxide poisoning occurs, it can have significant and even fatal consequences for aircraft occupants.
Carbon Monoxide Poisoning Accidents
1. On the morning of November 8,1957, Pan Am Flight 7, a Boeing 377, departed San Francisco on route to Honolulu. The last contact was a radio transmission to a US Coast Guard, however the flight never reached Hawaii. Post Mortem suggested higher than normal levels of Carbon Monoxide and it was suggested that carbon monoxide poisoning might have been a factor in the crash.
2. Carbon Monoxide Poisoning is very relevant to pilots flying lighter aircrafts i.e Piper, Diamond Aircrafts. Recently, in 2010, two people died in Hampshire due to Carbon Monoxide poisoning. A pathologist concluded that the level of carbon monoxide in the pilots blood, resulted in possibly “ a severe headache, nausea and grogginess to the extent where the pilot’s judgment and performance may have been compromised”
Therefor its important to understand the Human Body, what carbon monoxide does to it, and lastly how to ensure it does not happen to you.
Why Carbon Monoxide poisoning should concern pilots?
Analysis of toxicology samples from fatal U.S. aircraft accidents between 1967 and 1993 showed that at least 360 victims had been exposed to Carbon Monoxide that impaired their abilities. This shows that Carbon Monoxide is a threat and one that pilots and passengers alike should be able to recognize and rectify.
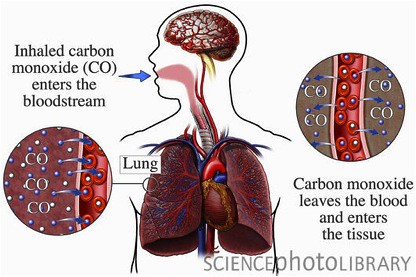
What is Carbon Monoxide?
Carbon Monoxide is a colorless, odorless and tasteless gas contained in exhaust fumes. Oxygen is delivered by hemoglobin, a molecule found in our red blood cells. In normal circumstances, oxygen that we breathe into our lungs is deposited on the hemoglobin molecules as blood passes through the lungs. The heart pumps the O2 rich blood to the tissues of the body where the oxygen is unloaded to aid as a critical component to help our body’s cells metabolize glucose.When breathed even in minute quantities over a period of time, it can reduce the ability of the blood to carry oxygen. Basically, it would block oxygen transport to tissues and eventually to organs like the heart. Subsequently, effects of hypoxia can occur.
How does CO get into the Cabin?
The exhaust of piston powered aircraft engines contains high concentrations of CO, particularly at mixtures settings rich of peak EGT. Most piston aircraft obtain heat by routing fresh air over surfaces of the muffler. When cabin heat is used, any cracks or holes in the muffler case can allow CO rich exhaust gas to contaminate the cabin air.
Other possible causes include inadequate sealing of the firewall, wheel wells or other air leaks that allows exhaust to leak into the cabin.
Signs and Symptoms
How to protect yourself?
The best protection against carbon monoxide is to avoid exposure.
Ensure that aircraft heating/ventilation systems and exhaust manifolds are in good working order.
Special attention should be paid to older aircrafts because of corrosion or simple or wear or tear.
- Turn the cabin heat fully off.
- Increase the rate of cabin fresh air ventilation to the maximum.
- Open windows if the flight profile and aircraft’s operating manual permit such an action.
- If available (provided it does not represent a safety or fire hazard), consider using supplemental oxygen.
- Land as promptly as possible.
- Do not hesitate to let Air Traffic Control know of your concerns, and ask for vectors to the nearest airport.
- Once on the ground, seek medical attention.
- Before continuing the flight, have the aircraft inspected by a certified mechanic.
Reference:
http://www.sciencephoto.com/media/120528/enlarge
Human Performance. (2009). Massey University (190.107) .
Authors / Editors
-Jagdeep Kang